 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free! Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
| Cat. No. | Especies | Descripción del producto | Estructura | Pureza | Característica |
|---|---|---|---|---|---|
| ILA-C52H8 | Cynomolgus | Cynomolgus IL-2 R alpha / CD25 Protein, His Tag (MALS verified) |  |
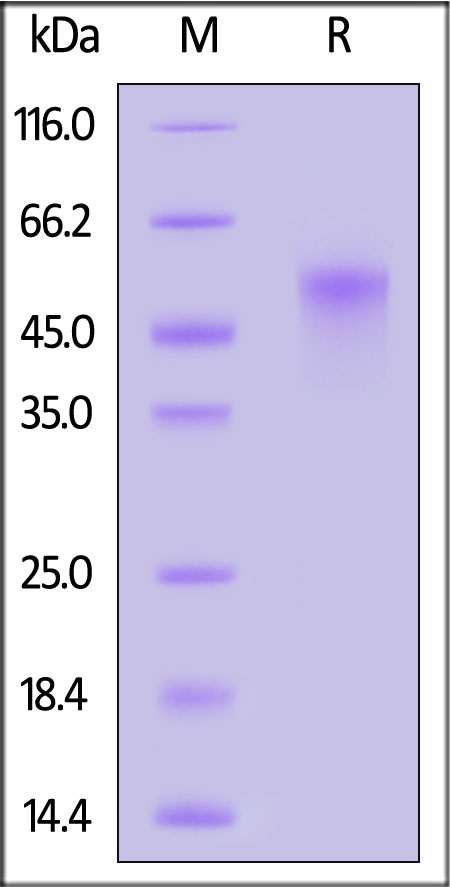
|
|
| ILA-H82F9 | Human | Biotinylated Human IL-2 R alpha / CD25 Protein, Fc,Avitag™ (MALS verified) |  |
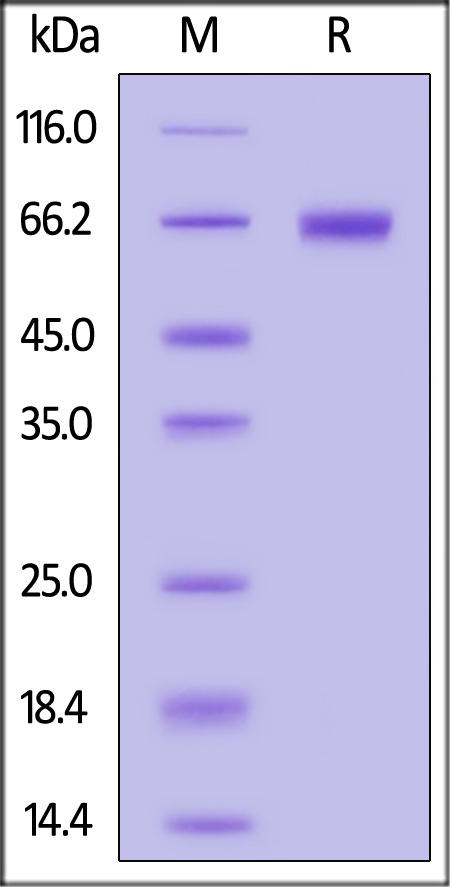
|
|
| ILA-C52H4 | Canine | Canine IL-2 R alpha / CD25 Protein, His Tag |  |
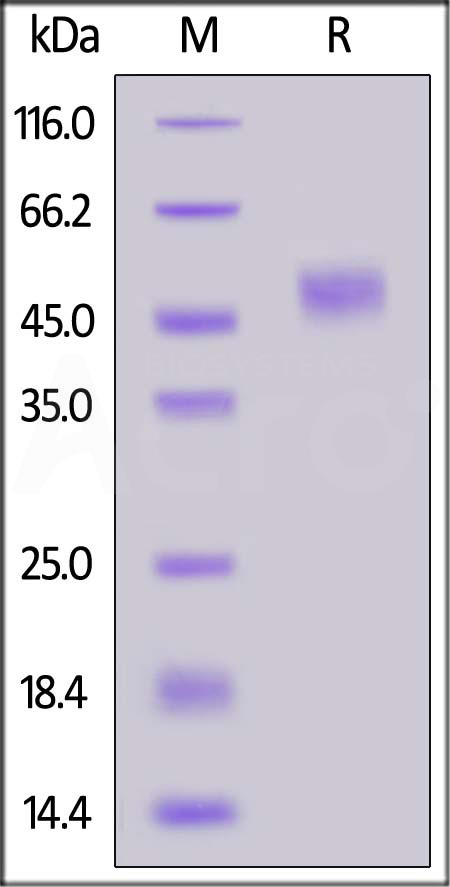
|
|
| ILA-M52H9 | Mouse | Mouse IL-2 R alpha / CD25 Protein, His Tag |  |
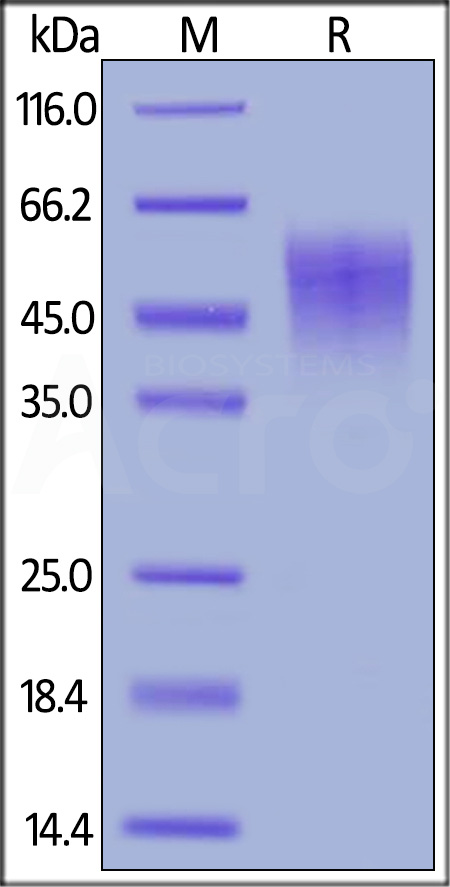
|
|
| ILA-H82E6 | Human | Biotinylated Human IL-2 R alpha / CD25 Protein, His,Avitag™, premium grade |  |
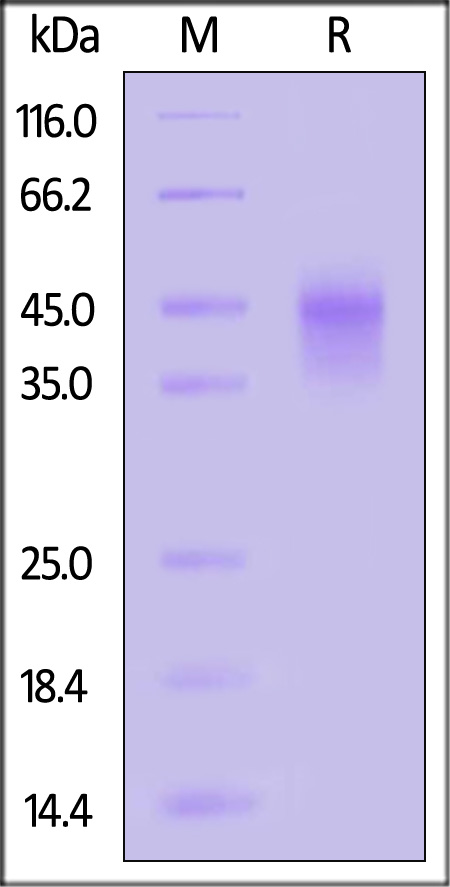
|
|
| ILA-H52H9 | Human | Human IL-2 R alpha / CD25 Protein, His Tag |  |
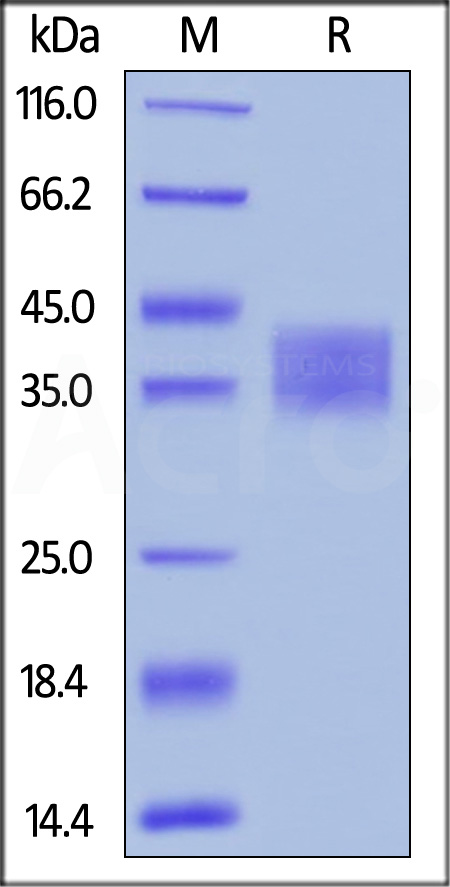
|
|
| ILA-H5251 | Human | Human IL-2 R alpha / CD25 Protein, Fc Tag (MALS verified) |  |

|

Biotinylated Human IL-2 R alpha, His,Avitag, premium grade (Cat. No. ILA-H82E6) inhibits the IL-2-dependent proliferation of Mo7e cells. The EC50 for this effect is 0.57-0.81 µg/mL (Routinely tested).

Human IL-2 R alpha, His Tag (Cat. No. ILA-H52H9) captured on CM5 chip via anti-His antibody, can bind Human IL-2, Tag Free with an affinity constant of 29.9 nM as determined in a SPR assay (Biacore T200) (Routinely tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Daclizumab biosimilar (Shanghai CP Guojian) | Approved | Sunshine GuoJian Pharmaceutical (Shanghai) Co Ltd | 健尼哌 | Mainland China | Rejection of renal transplantation | null | 2011-01-12 | Rejection of renal transplantation | Details | |
| Daclizumab | R-35; BIIB-019; RO-247375 | Approved | Abbvie Inc, Biogen Inc, F. Hoffmann-La Roche Ltd | Zinbryta, Zenapax | United States | Rejection of organ transplantation | F. Hoffmann-La Roche Ag | 1997-12-10 | Arthritis, Juvenile; Leukemia, T-Cell; Behcet Syndrome; Melanoma; Lymphoma; Iritis; Uveitis; Thrombocytopenia; Rejection in heart transplantation; Uveitis, Anterior; Colitis, Ulcerative; Asthma; Breast Neoplasms; Psoriasis; Multiple Sclerosis, Relapsing-Remitting; Retinal Diseases; Radiation Injuries; Carcinoma, Ductal; Rejection of organ transplantation; Multiple Sclerosis; HTLV-I Infections; Myelodysplastic Syndromes; Gastrointestinal Diseases; Inflammatory Bowel Diseases; Leukemia; Granulomatosis with Polyangiitis; HIV Infections; Diabetes Mellitus, Type 1 | Details |
| Basiliximab | CHT-25; chRFT5; CHI-621; SDZ-CHI-621 | Approved | Novartis Pharma Ag | 舒莱, Simulect | United States | Rejection of organ transplantation | Novartis Pharma Ag | 1998-05-12 | Anemia, Aplastic; Leukemia, Lymphocytic, Chronic, B-Cell; Kidney Failure, Chronic; Uveitis; Lymphoma, Non-Hodgkin; Leukemia, Myeloid, Acute; Emphysema; Pulmonary Disease, Chronic Obstructive; Colitis, Ulcerative; Primary Myelofibrosis; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Rejection of renal transplantation; Multiple Myeloma; Rejection of organ transplantation; Myelodysplastic Syndromes; Coronavirus Disease 2019 (COVID-19); Hodgkin Disease; Kidney Diseases; Keratoplasty rejection; Hemoglobinuria, Paroxysmal; Cytokine Release Syndrome; Leukemia, Myelogenous, Chronic | Details |
| Daclizumab biosimilar (Shanghai CP Guojian) | Approved | Sunshine GuoJian Pharmaceutical (Shanghai) Co Ltd | 健尼哌 | Mainland China | Rejection of renal transplantation | null | 2011-01-12 | Rejection of renal transplantation | Details | |
| Daclizumab | R-35; BIIB-019; RO-247375 | Approved | Abbvie Inc, Biogen Inc, F. Hoffmann-La Roche Ltd | Zinbryta, Zenapax | United States | Rejection of organ transplantation | F. Hoffmann-La Roche Ag | 1997-12-10 | Arthritis, Juvenile; Leukemia, T-Cell; Behcet Syndrome; Melanoma; Lymphoma; Iritis; Uveitis; Thrombocytopenia; Rejection in heart transplantation; Uveitis, Anterior; Colitis, Ulcerative; Asthma; Breast Neoplasms; Psoriasis; Multiple Sclerosis, Relapsing-Remitting; Retinal Diseases; Radiation Injuries; Carcinoma, Ductal; Rejection of organ transplantation; Multiple Sclerosis; HTLV-I Infections; Myelodysplastic Syndromes; Gastrointestinal Diseases; Inflammatory Bowel Diseases; Leukemia; Granulomatosis with Polyangiitis; HIV Infections; Diabetes Mellitus, Type 1 | Details |
| Basiliximab | CHT-25; chRFT5; CHI-621; SDZ-CHI-621 | Approved | Novartis Pharma Ag | 舒莱, Simulect | United States | Rejection of organ transplantation | Novartis Pharma Ag | 1998-05-12 | Anemia, Aplastic; Leukemia, Lymphocytic, Chronic, B-Cell; Kidney Failure, Chronic; Uveitis; Lymphoma, Non-Hodgkin; Leukemia, Myeloid, Acute; Emphysema; Pulmonary Disease, Chronic Obstructive; Colitis, Ulcerative; Primary Myelofibrosis; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Rejection of renal transplantation; Multiple Myeloma; Rejection of organ transplantation; Myelodysplastic Syndromes; Coronavirus Disease 2019 (COVID-19); Hodgkin Disease; Kidney Diseases; Keratoplasty rejection; Hemoglobinuria, Paroxysmal; Cytokine Release Syndrome; Leukemia, Myelogenous, Chronic | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Inolimomab | B-B10; BT-563; MAb-BT-563 | Phase 3 Clinical | Eusa Pharma, Jazz Pharmaceuticals Plc | Graft vs Host Disease | Details |
| Nemvaleukin alfa | RDB-1419; RDB-1450; ALKS-4230 | Phase 3 Clinical | Alkermes Plc | Ovarian Neoplasms; Solid tumours; Skin Melanoma; Squamous Cell Carcinoma of Head and Neck; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Melanoma | Details |
| Basiliximab biobetter (Mabtech/Sorrento) | STI-003 | Phase 3 Clinical | Taizhou Maibo Taike Biotechnology Co Ltd | Autoimmune Diseases | Details |
| PT-101 | PT-101; MK-6194; PT101 | Phase 2 Clinical | Pandion Therapeutics | Lupus Erythematosus, Systemic; Colitis, Ulcerative; Dermatitis, Atopic; Vitiligo | Details |
| LMB-2 | LMB-2; LMB-2a | Phase 2 Clinical | National Cancer Institute | Leukemia; Leukemia, Hairy Cell; Myeloproliferative Disorders; Myelodysplastic Syndromes; Skin Neoplasms; Leukemia-Lymphoma, Adult T-Cell; Lymphoma; Myelodysplastic-Myeloproliferative Diseases; Melanoma | Details |
| Camidanlumab tesirine | ADCT-301 | Phase 2 Clinical | Adc Therapeutics Sa, Genmab A/S | Myeloproliferative Disorders; Melanoma; Carcinoma, Non-Small-Cell Lung; Fallopian Tube Neoplasms; Leukemia, Myeloid, Acute; Lymphoma, Non-Hodgkin; Colorectal Neoplasms; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Urinary Bladder Neoplasms; Myelodysplastic Syndromes; Ovarian Neoplasms; Pancreatic Neoplasms; Triple Negative Breast Neoplasms; Hodgkin Disease; Carcinoma, Renal Cell; Stomach Neoplasms; Esophageal Neoplasms; Squamous Cell Carcinoma of Head and Neck; Solid tumours | Details |
| 90Y Basiliximab | Phase 2 Clinical | City Of Hope National Medical Center | Lymphoma, T-Cell, Peripheral; Hodgkin Disease; Myelodysplastic Syndromes; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, Myeloid, Acute; Lymphoma, T-Cell, Cutaneous; Lymphoma, Non-Hodgkin; Lymphoma, T-Cell | Details | |
| MDNA-11 | MDNA-11; MDNA11 | Phase 2 Clinical | Medicenna Therapeutics Corp | Triple Negative Breast Neoplasms; Melanoma; Uterine Cervical Neoplasms; Carcinoma, Non-Small-Cell Lung; Fallopian Tube Neoplasms; Endometrial Neoplasms; Carcinoma, Squamous Cell; Peritoneal Neoplasms; Mesothelioma; Urinary Bladder Neoplasms; Skin Neoplasms; Solid tumours; Carcinoma, Ovarian Epithelial; Stomach Neoplasms; Carcinoma, Merkel Cell; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Renal Cell; Esophageal Neoplasms; Gastrointestinal Diseases; Carcinoma, Basal Cell; Ovarian Neoplasms; Skin Melanoma | Details |
| Anti-interleukin-2 receptor monoclonal antibody Mikbeta1 | HuMikβ1-1 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd, National Institutes Of Health | Leukemia; Leukemia, Lymphoid; Celiac Disease; Paraparesis, Tropical Spastic; Leukemia, Large Granular Lymphocytic | Details |
| Vopikitug | RO-7296682; RG-6292 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Solid tumours; Neoplasms | Details |
| BA-1106 | TS-1904; BA-1106; RR-102 | Phase 1 Clinical | Solid tumours; Neoplasms | Details | |
| XmAb-564 | XmAb-564; XmAb-27564 | Phase 1 Clinical | Xencor Inc | Autoimmune Diseases; Psoriasis; Dermatitis, Atopic | Details |
| IL2 CD25 fusion protein (Bristol-Myers Squibb) | Phase 1 Clinical | University Of Miami | Autoimmune Diseases | Details | |
| 9MW-3911 | 9MW3911; 9MW-3911 | Phase 1 Clinical | Nanjing Nuo Aixin Biotechnology Co Ltd | Neoplasms | Details |
| In 111-DOTA-Basiliximab | Phase 1 Clinical | City Of Hope National Medical Center | Myelodysplastic Syndromes; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, Myeloid, Acute | Details | |
| BMS-986326 | BMS-986326 | Phase 1 Clinical | Bristol-Myers Squibb Company | Lupus Vulgaris; Dermatitis, Atopic | Details |
| Humanized Anti-CD25 Antibody(Institute of Hematology & Blood Diseases Hospital) | Institute Of Hematology & Blood Diseases Hospital | Details | |||
| Inolimomab | B-B10; BT-563; MAb-BT-563 | Phase 3 Clinical | Eusa Pharma, Jazz Pharmaceuticals Plc | Graft vs Host Disease | Details |
| Nemvaleukin alfa | RDB-1419; RDB-1450; ALKS-4230 | Phase 3 Clinical | Alkermes Plc | Ovarian Neoplasms; Solid tumours; Skin Melanoma; Squamous Cell Carcinoma of Head and Neck; Peritoneal Neoplasms; Fallopian Tube Neoplasms; Melanoma | Details |
| Basiliximab biobetter (Mabtech/Sorrento) | STI-003 | Phase 3 Clinical | Taizhou Maibo Taike Biotechnology Co Ltd | Autoimmune Diseases | Details |
| PT-101 | PT-101; MK-6194; PT101 | Phase 2 Clinical | Pandion Therapeutics | Lupus Erythematosus, Systemic; Colitis, Ulcerative; Dermatitis, Atopic; Vitiligo | Details |
| LMB-2 | LMB-2; LMB-2a | Phase 2 Clinical | National Cancer Institute | Leukemia; Leukemia, Hairy Cell; Myeloproliferative Disorders; Myelodysplastic Syndromes; Skin Neoplasms; Leukemia-Lymphoma, Adult T-Cell; Lymphoma; Myelodysplastic-Myeloproliferative Diseases; Melanoma | Details |
| Camidanlumab tesirine | ADCT-301 | Phase 2 Clinical | Adc Therapeutics Sa, Genmab A/S | Myeloproliferative Disorders; Melanoma; Carcinoma, Non-Small-Cell Lung; Fallopian Tube Neoplasms; Leukemia, Myeloid, Acute; Lymphoma, Non-Hodgkin; Colorectal Neoplasms; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Urinary Bladder Neoplasms; Myelodysplastic Syndromes; Ovarian Neoplasms; Pancreatic Neoplasms; Triple Negative Breast Neoplasms; Hodgkin Disease; Carcinoma, Renal Cell; Stomach Neoplasms; Esophageal Neoplasms; Squamous Cell Carcinoma of Head and Neck; Solid tumours | Details |
| 90Y Basiliximab | Phase 2 Clinical | City Of Hope National Medical Center | Lymphoma, T-Cell, Peripheral; Hodgkin Disease; Myelodysplastic Syndromes; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, Myeloid, Acute; Lymphoma, T-Cell, Cutaneous; Lymphoma, Non-Hodgkin; Lymphoma, T-Cell | Details | |
| MDNA-11 | MDNA-11; MDNA11 | Phase 2 Clinical | Medicenna Therapeutics Corp | Triple Negative Breast Neoplasms; Melanoma; Uterine Cervical Neoplasms; Carcinoma, Non-Small-Cell Lung; Fallopian Tube Neoplasms; Endometrial Neoplasms; Carcinoma, Squamous Cell; Peritoneal Neoplasms; Mesothelioma; Urinary Bladder Neoplasms; Skin Neoplasms; Solid tumours; Carcinoma, Ovarian Epithelial; Stomach Neoplasms; Carcinoma, Merkel Cell; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Renal Cell; Esophageal Neoplasms; Gastrointestinal Diseases; Carcinoma, Basal Cell; Ovarian Neoplasms; Skin Melanoma | Details |
| Anti-interleukin-2 receptor monoclonal antibody Mikbeta1 | HuMikβ1-1 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd, National Institutes Of Health | Leukemia; Leukemia, Lymphoid; Celiac Disease; Paraparesis, Tropical Spastic; Leukemia, Large Granular Lymphocytic | Details |
| Vopikitug | RO-7296682; RG-6292 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Solid tumours; Neoplasms | Details |
| BA-1106 | TS-1904; BA-1106; RR-102 | Phase 1 Clinical | Solid tumours; Neoplasms | Details | |
| XmAb-564 | XmAb-564; XmAb-27564 | Phase 1 Clinical | Xencor Inc | Autoimmune Diseases; Psoriasis; Dermatitis, Atopic | Details |
| IL2 CD25 fusion protein (Bristol-Myers Squibb) | Phase 1 Clinical | University Of Miami | Autoimmune Diseases | Details | |
| 9MW-3911 | 9MW3911; 9MW-3911 | Phase 1 Clinical | Nanjing Nuo Aixin Biotechnology Co Ltd | Neoplasms | Details |
| In 111-DOTA-Basiliximab | Phase 1 Clinical | City Of Hope National Medical Center | Myelodysplastic Syndromes; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, Myeloid, Acute | Details | |
| BMS-986326 | BMS-986326 | Phase 1 Clinical | Bristol-Myers Squibb Company | Lupus Vulgaris; Dermatitis, Atopic | Details |
| Humanized Anti-CD25 Antibody(Institute of Hematology & Blood Diseases Hospital) | Institute Of Hematology & Blood Diseases Hospital | Details |
This web search service is supported by Google Inc.
